Australia & New Zealand
Dear Customer,
We’ve been informed that a paper was recently circulated in Australia by a local “scientist” claiming the Ganshorn ScoutTube, promoted by Bird Healthcare, is compatible with NDD devices. NDD has serious concerns about the data presented in this paper and its conclusions.
Specifically:
- The paper wasn’t dated or officially published as a scientific paper, nor was it peer-reviewed by independent scientists (officially published meaning through a recognised magazine, journal or Scientific Conference)
- The paper was not submitted to ERS as a “virtual poster” as claimed by the author
- The testing methodology used was unclear and lacks statistical significance. Only one ScoutTube was tested.
- The lone individual circulating this paper is connected commercially to Bird Healthcare. (He is a co-director of a respiratory company based in Melbourne that also has Directors from Bird Healthcare listed on it’s board.)
Hans Rudolph Inc., an accredited independent testing laboratory not associated with NDD, also tested the Ganshorn ScoutTube. Their report shows that the ScoutTube is not compliant with ERS / ATS guidelines regarding accuracy and repeatability.
Here are the summary results of that report:
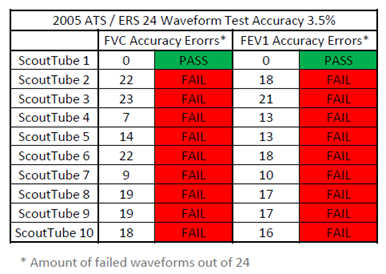
- Hans Rudolph tested 10 ScoutTubes, but only one (1) of the ScoutTubes passed the 24 waveform test according to the 2005 ATS /ERS guidelines. 9 out of 10 ScoutTubes failed the test (please see chart below). Please also note that the test result variability between different ScoutTubes in the sample is quite large.
- If more rigorous NDD specifications are applied to the data, i.e., accuracy must be within 3%, then 9 out of 10 ScoutTubes will fail additional waveform tests.
Given this independent data, NDD cannot recommend the use of the Ganshorn ScoutTubes with NDD devices. Using non-compliant equipment and risking inaccurate PFT tests is not worth the small potential for costs savings if any, by using non approved accessories. Instead, we recommend using safer NDD solutions because they are:
- Compliant with ATS/ERS guidelines
- Tested via rigorous, statistically significant methods and via accredited independent testing laboratories
- Custom engineered to fit precisely with NDD devices
If you would like to review the independent Hans Rudolph report in detail, or if you would like to speak with NDD about our PFT testing solutions and accessories, please fill out the form and we’ll contact you directly. Thank you for your time and attention.
George Harnoncourt
CEO, NDD Medical Technologies

