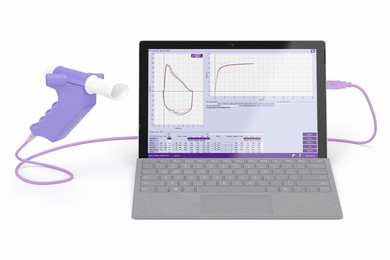
For those searching for a fully-integrated PC-driven spirometer, the Easy on-PC is a clinically proven solution. The Easy-on PC leverages our trusted TrueFlow™ technology that delivers accuracy and reliable results without needing calibration. Real-time graphs and animated incentive screens enhance the user experience, encouraging child and adult patients to help them achieve optimal results.
- Calibration-free & Quick Spirometry
TrueFlow™ technology, high-quality & optimized components and Swiss precision-manufacturing deliver quick & accurate results without calibration.
- Quality Feedback & Interpretation
Simplify testing using the instant test quality feedback and interpretation platform.
- Real-Time Animated Graphs and Incentives
Interactive software displays, animated incentive screens and real-time graphs encourage child and adult patients to help them achieve maximum results.
- EMR Connectivity & Customized Settings
The flexibility of settings permits customized solutions including EMR/EHR integration to meet your individual needs.
"My clinical research on asthma and COPD happens at the point of care in some of the most remote, resource limited places in the world. Because of this, I need highly portable, very durable diagnostic devices reporting consistent and accurate results even under the toughest conditions. I use ndd’s devices because I know no matter the condition, the device will report accurate results."William Checkley, MDAssociate professor at John Hopkins Bloomberg School of Public Health
"Our academic activities related to trainings and continuous learning for the specialists that take care of the respiratory health in our region, are supported by new technologies. Our experience with ndd devices has been very positive because they adapt to our necessities, they are always updated regarding new standardization and technological advances."Gustavo GiraldoPublic Relations and Marketing Manager/Asociación Latinoamericana de Tórax (ALAT)
Details Easy on-PC
Research studies show reliable sensor performance under various conditions
Get optimal results with Easy on-PC, which is designed for maximum robustness under various conditions.
- Highly resistant to shock and vibration
- Strong cable protection
- Works in diverse environments
- Completely calibration-free

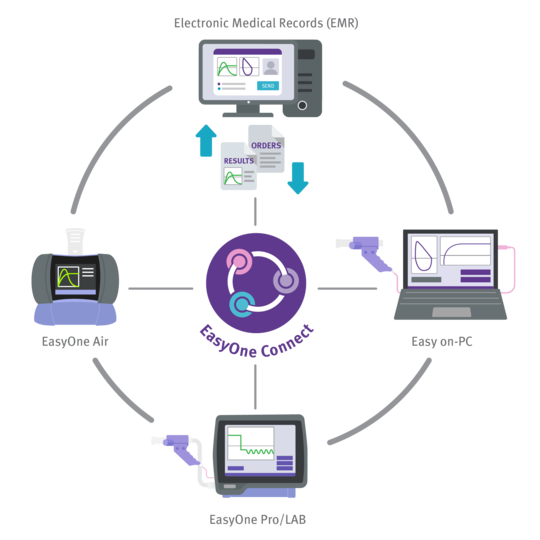
Powered by EasyOne Connect - ndd's Integrated Software Platform
The Easy on-PC spirometry sensor is directly connected via USB to your workstation where the EasyOne Connect software is installed. This software includes all advanced spirometry functionality at no extra charge and fulfills ATS/ERS 2005 and 2019 standards. Features include:
- QC-grading and result interpretation
- Powerful commenter functionality
- Encouraging pediatric incentives
- Customized reports
- Custom provocation protocols
- Large selection of predicted sets (%Pred, Z-score, and LLN)
- Import of external PFT results
For more information, please visit the EasyOne Connect product page.

Powerful EMR EHR Integration Engine
EasyOne Connect software includes powerful EMR EHR interoperability and IT management functionality for streamlined workflows. Features include:
- Proven EMR integrations
- Supports integration standards - HL7, XML, API, GDT, etc.
- Active directory integration
- SQL Server database
For more information, please visit the EMR EHR interoperability solutions page.
Worldwide Compliance with Standards
Stay compliant with ATS/ERS standards and specific country regulations.
- Fully conformant to ATS/ERS 2005 and 2019 and ISO standards
- Worldwide approvals
- CE Approval
- FDA Approval
- China Approval
Click here for a complete list of ndd certificates.

TrueFlow™ - Trusted Ultrasound Technology
Stop worrying about calibration or accuracy of flow measurements. TrueFlow™ is the only ultrasound technology proven to be accurate for a lifetime for flow and volume measurements.
- Proven long-term stability
- Contact and resistance-free measurement
- Excellent accuracy and robustness
- Patented technology
To review case studies and additional information about our proven technology, please click here.
Infection Control
The EasyOne Filter solutions provide additional protection for those who wish to include a filter when performing tests.
- The EasyOne Filter keeps the ambient environment clean for technicians and patients.
- The ndd breathing mouthpiece protects the flow sensor from contamination.
- Fully passes 13 Waveform test as required by the 2019 ATS/ERS standard.
- Available for most testing environments where filters are required.
Avoid cross-contamination and keep cleaning to an absolute minimum:
- All parts exposed to the patient's breath are single-patient-use.
- The sensor is protected from contamination by the ndd breathing mouthpiece.
- Only simple surface cleaning is required for the ndd device.
- No special storage conditions are required for single-patient use Spirettesand FlowTubes (ndd breathing mouthpieces).
Visit our Infection Control page for more information.
Software releases Easy on-PC
EasyOne Connect V03.09.05.19
FAQs Easy on-PC
ndd can integrate with any EHR vendor that can support a bi-directional HL7 orders/results lab interface.
ndd offers a file-based integration utilizing an SFTP or network-based location for file communication with an EHR vendor. HL7 is the standard data structure that ndd utilizes but can support other data formats as well. The use of other interface engines can be utilized to complete an integration project.
The first step for an integration project is to complete the EMR integration request form here: https://nddmed.com/emr. An integration specialist will then reach out to you with the next steps.
Duration depends on the IT team of the facility. The ndd side of the integration process can be completed within a few hours for standard integrations. EasyOne Connect software is simply configured to point to the SFTP or network-based location where files are being placed.
EasyOne Connect software utilizes AES 256 encryption. SFTP has its own security protocols built into it. If a network-based location is utilized, it will follow the protocols of the facility.
Yes, EasyOne Connect offers an optional centralized patient database feature, enabling seamless access to patient orders and results across all instances of the software, irrespective of physical location. Customers can leverage their on-premise SQL servers or MS Azure environments to ensure data accessibility and consistency.
EasyOne Connect provides flexible options for managing users and their access. User accounts can be created and managed directly within EasyOne Connect for simple permission control. For more advanced management, the software supports integration with Active Directory for using your existing directory service accounts, groups, and permissions. EasyOne Connect also supports optional single sign-on (SSO) capability through integration with major identity providers. Please see application note for additional information.
ndd has integrated with all major vendors and can provide examples of successful integrations with various healthcare providers and organizations. You can contact our integration department via email at [email protected] for more information.
ndd does not handle the maintenance and upgrades as the software is installed locally on the client’s machine. We do, however, offer a silent installer package that our support team can assist you with. This silent installer can be executed via a script through active directory commands depending on your facility. It is the responsibility of the facility to implement any future updates. There is no licensing fee associated with the software and/or the updates.
ndd does not charge for standard integration. In the event custom development is needed, ndd will provide a statement of work before a project begins. Almost all EHR vendors will charge a fee that the client is responsible for.
HL7 specifications can be found here.
- Local SQLite file-based database.
- On-premise Microsoft SQL Server database.
- Azure cloud-hosted Microsoft SQL Server database.
Please see the application note for additional information.
The facility is responsible for setting up and hosting either option. ndd requires a username and password if using an SFTP environment.
The result HL7 message will consist of a combination of discrete results and an embedded base64 PDF attachment for the PFT report. If you prefer, we also offer the option of including a reference link instead of the embedded PDF.
No, a server is not required.
You can schedule a meeting with an integration engineer from 8 a.m. to 5 p.m. ET Monday through Friday. ndd can accommodate meetings outside of that timeframe if planned accordingly. The integration team can be reached via email at [email protected]. The support team can also be reached via email at [email protected] or by phone at (978) 470-0923.
You can find the product specification on the respective product pages. The specification contains a list of all supported normal values.
To get more detailed information about the supported parameters, age range, ethnicities and height range of each supported predicted set, please click here.
BTPS Correction is used to convert flow and volume measured at ambient conditions to the conditions within the lungs. Ambient conditions are called ATP (ambient temperature, pressure); the conditions within the lungs are called BTPS (body temperature, pressure, water vapor saturated).
Click here for more information.
The Easy on-PC software, EasyOne Connect, can be downloaded free of charge directly from our website. Click here for the download.
Yes, the EasyOne Connect software can be downloaded onto as many laptop, PC or tablet devices as desired. There are no license requirements.
No, a laptop, PC or tablet must be purchased separately. The device specifications can be found in the Requirements PC/Laptop section under Specifications on the Easy on-PC page.
Please consult Section 1.11 in the manual here for the latest computer requirements. Note that for the Easy on-PC latest version, V02.02.00.14 or greater, the following operating systems are no longer supported: Windows XP SP3 and Windows Vista.
The general requirements for notebooks and tablets the following:
- USB interface
- Windows operating system (32 or 64 bit)
- Hard disk capacity of 1 GB installation and up to 4 GB of data
- RAM 2 GB
The embedded versions of Windows are no longer compatible.
The EasyOne Connect software for the Easy on-PC offers a variety of animated screens for children as well as adults. These incentive screens, in addition to the real-time curves, encourage the patients to help them achieve high quality spirometry results. Here are some examples of the pediatric incentive screens available:

Data can safely be exported by using the “Export Data” feature or “Backup” feature within the Storage tab in the configuration.
All our products can be purchased through our authorized distribution partners. For US customers, please contact us to receive more information on finding your local dealer. International customers please click here for a list of dealers in your country.
No. All EasyOne products are designed to not require calibration. ndd’s patented TrueFlow technology is the only technology proven to remain stable over its lifetime. A number of independent papers have been published that confirm this long-term stability.
For organizations like NIOSH/OSHA or Social Security/Disability, where calibration checks are required, ndd offers a 3-Liter calibration syringe. A calibration check (cal-check) is not to be confused with calibration. A cal-check simply validates that the device is within calibration limits. Unlike calibration, a cal-check does not adjust the calibration values.
Specifications Easy on-PC
Installation/ system 1 GB
Data up to 4 GB